News
KYOCERA Medical Corporation Receives FDA 510(k) Clearance for Initia® Total Hip System, featuring BIOCERAM AZUL® Ceramic Femoral Head
San Diego, CA — Dec. 5, 2016 — Kyocera Medical Corporation, a leading manufacturer of implantable systems and advanced ceramic components,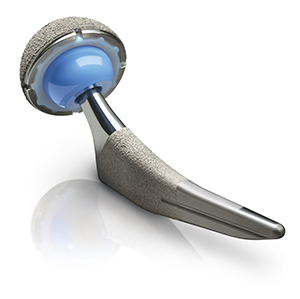 today announced it has received the U.S. Food and Drug Administration’s 510(k) clearance for its Initia® Total Hip System.
today announced it has received the U.S. Food and Drug Administration’s 510(k) clearance for its Initia® Total Hip System.
Developed in collaboration with leading U.S. surgeons, the Initia Total Hip System features Kyocera Medical Corporation’s core technologies — including its own BIOCERAM AZUL® (blue) zirconia-toughened alumina (ZTA) ceramic femoral heads. Initia is also available with cobalt chrome (CoCr) femoral heads, giving surgeons the option of ceramic or metal heads matched to highly cross-linked polyethylene acetabular liners. Designed for a global patient population, the Initia system includes a total of 16 tapered-wedge stem sizes, 12 of which are available in the U.S., with both standard and high femoral offsets.
Kyocera has manufactured artificial hips, knees and other implantable systems since 1982, and is now one of Japan’s leading suppliers of surgical orthopedic products. Kyocera also manufactures a wide range of implantable ceramic components, including alumina and ZTA ceramic femoral heads, hip liners and shoulder liners available to original equipment manufacturers (OEMs) in the U.S. and Europe.
In addition to the Initia Total Hip System, Kyocera Medical Corporation’s BIOCERAM AZUL ceramic material is available to medical device OEMs who demand a durable, high-quality, biocompatible solution for implantable components.
The Initia Hip System, along with BIOCERAM AZUL components, will be the highlight of Kyocera Medical Corporation’s technology presentation at the AAOS Annual Meeting, March 15-17, 2017 – booth #4908.
ABOUT KYOCERA
Kyocera International, Inc., headquartered in San Diego, California, serves as the U.S. sales and marketing arm for Kyocera Medical Corporation. Both companies are wholly-owned subsidiaries of Kyoto, Japan-based Kyocera Corporation.
Kyocera Medical Corporation, headquartered in Osaka, Japan, is a producer of ceramic and metal orthopedic systems and components, dental systems and components, and cardiovascular devices.
Kyocera Corporation (NYSE:KYO) (TOKYO: 6971), the parent and global headquarters of the Kyocera Group, was founded in 1959 as a producer of fine ceramics (also known as “advanced ceramics”). Kyocera specializes in combining these engineered materials with other technologies to create medical orthopedic implants and implantable ceramic components, ceramic cutlery and cookware, cutting tools, industrial components, electronic devices, semiconductor packages, solar power generating systems, printers, copiers and mobile phones. During the year ended March 31, 2016, Kyocera Corporation’s consolidated net sales totaled $13.1 billion.
Contact: Ken Kaneko, Ken.Kaneko@kyocera.com, (908) 227-4376; Photos available by request.
© 2016 KYOCERA Corporation. All rights reserved. Kyocera is a registered trademark of Kyocera Corporation. Initia and BIOCERAM AZUL are registered trademarks of Kyocera Medical Corporation. All other marks are properties of their respective owners. Photo © Kyocera Medical Corporation.
# # #

